Abstract
Background Patients with newly diagnosed AML can often present with debilitating medical conditions, active infection, concurrent malignancies, significant cardiovascular disease, and poor performance status. There is a lack of trial options for these patients. Such patients would have been excluded from the VIALE-A trial, that studied HMA + venetoclax in older and unfit patients. Frontline cladribine (Clad) with low dose cytarabine (LDAC) alternating with decitabine yielded an overall response rate (ORR) of 68%, a median overall survival (OS) 13.8 months, and was well-tolerated, with a 4-week mortality of 1% in an older patient population. This combination potentially provides an avenue for therapy in an unfit patient population with significant comorbidities. Here we present the results of this phase II study in unfit patients, ineligible for other clinical trials.
Methods Pts over 18 with newly diagnosed AML found to be ineligible for clinical trials due to severe comorbidity, active infection/malignancy, or poor PS were enrolled. To be eligible, pts must have at least 1 of the following: Cr ≥2mg/dL, Bilirubin ≥2 mg/dL, ECOG of 3 or 4, active infection, concurrent active malignancy, or ineligibility for a higher priority protocol. Induction consisted of Clad 5mg/m2 on D1-5 and LDAC 20mg SQ BID on D1-10. Consolidation consisted of Clad 5mg/m2 on D1-3 and LDAC 20mg SQ BID on D1-10 with pts receiving decitabine 20mg/m2 IV monotherapy on D1-5 on alternate cycles. The primary objective was 60-day mortality.
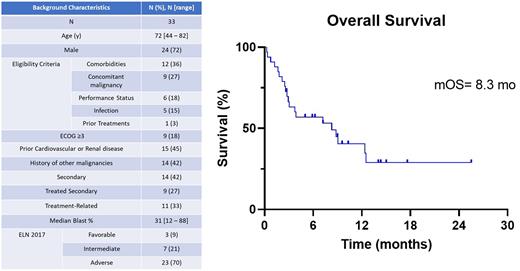
Results Between 04/2020 and 05/2022, 33 pts have been enrolled. Baseline characteristics are in Table 1. The median age is 72 yrs (range: 44-82). Fulfilled eligibility criteria for study entry included: comorbidities [acute or chronic renal dysfunction in 7 (21%) pts, cardiac in 2 (6%) , hepatic in 2 (6%), and morbid obesity with advanced PS in 1 (3%) pt] in 12 pts (36%) concomitant (active) malignancy in 9 (27%), poor PS in 6 (18%) pts , active infection in 5 pts (15%), and numerous prior treatments for MDS in 1 pt (3%), making them ineligible for other trials. 15 (45%) had history of ongoing cardiovascular or renal disease. 14 (42%) pts had history of other malignancies. 11 (33%) pts had therapy-related disease. The median blast percent was 31% (range: 12-88). Adverse risk disease based on ELN 2017 criteria was overrepresented in the cohort, occurring in 70% of pts. Complex karyotype and TP53 mutations occurred in 18% and 21% of pts, respectively.
The overall response rate (CR/CRi/PR) was 66% (22 pts) with a CR/CRi rate of 63% (21) and a CR rate of 33% (11). 30% (10) attained an MRD negative remission. The median cycles to response was 1 (range: 1-4). Of the pts that responded, 3 (9%) remain on-trial, 2 (6%) went on to allogeneic SCT, 5 (15%) proceeded to post-remission maintenance strategies, 5 (15%) relapsed, 5 (15%) died after infections, 1 (3%) died after falling and developing a subdural hematoma, and 1 (3) was taken off trial due to prolonged myelosuppression.
At a median follow up of 14 months, the median OS and median RFS were 8.3 months (95% CI: 0.19-15.7) and 7.9 months (95% CI: 0.45-15.3) respectively. The OS for pts with ECOG 1, 2 and >3 PS was 8.3, 7.6 and 8.8 months respectively. Pts with significant cardiac or renal disease had worse OS at 3.03 months compared to 12.5 months (p = 0.03) in pts without this history.
Grade ≥3 adverse events possibly related to this combination occurred in 2 patients: one with Grade 3 nausea and the second with Grade 3 tumor lysis syndrome. The 30-day and 60-day survival were 91% and 82%% respectively. Of the 6 patients (18%) that experienced 60-day mortality, 4 (12%) died from infection (2 with pneumonia and 2 with bacteremia), 1 (3%) patient died due to a heart failure exacerbation with a critical coronary artery stenosis, and 1 (3%) pt died after transitioning to another trial, developing a medullary lesion, and opting for hospice.
Conclusions In an unfit patient population with numerous comorbidities, the combination of Clad and LDAC was well tolerated with a 60-day survival of 82%, an ORR of 66%, and a median OS of 8.3 months. Once in remission, patients were able to move onto maintenance therapy or SCT in an improved condition. This combination represents a potential option for treatment in a high-risk, unfit, and undertreated patient population and demonstrates the feasibility of safely expanding trial inclusion criteria for select populations of patients.
Disclosures
Kantarjian:Daiichi-Sankyo: Consultancy, Research Funding; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Research Funding; NOVA Research: Honoraria; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Research Funding; ImmunoGen: Research Funding; KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Takeda: Honoraria. Jabbour:Spectrum: Research Funding; Genentech: Other: Advisory Role, Research Funding; Pfizer: Other: Advisory Role, Research Funding; Bristol Myers Squibb: Other: Advisory Role, Research Funding; Amgen: Other: Advisory Role, Research Funding; Adaptive Biotechnologies: Other: Advisory Role, Research Funding; AbbVie: Other: Advisory Role, Research Funding; Takeda: Other: Advisory Role, Research Funding. Alvarado:Jazz Pharmaceuticals: Research Funding; Daiichi-Sankyo/Lilly: Research Funding; BerGenBio: Research Funding; FibroGen: Research Funding; Astex Pharmaceuticals: Research Funding; Sun Pharma: Research Funding. Borthakur:Astex Pharmaceuticals, Ryvu, PTC Therapeutics: Research Funding; Catamaran Bio, Abbvie, PPD Development, Protagonist Therapeutics, Janssen: Consultancy; Pacylex, Novartis, Cytomx, Bio Ascend: Membership on an entity's Board of Directors or advisory committees. Issa:Celgene, Kura Oncology, Syndax, Merck, Cullinan and Novartis: Research Funding; Novartis, Kura Oncology, Nuprobe: Consultancy. Daver:Agios, Celgene, SOBI and STAR Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kartos and Jazz Pharmaceuticals: Other: Data monitoring committee member; Karyopham Therapeutics and Newave Pharmaceutical: Research Funding; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Novartis, Jazz, Amgen, Servier, Karyopharm, Trovagene, Trillium, Syndax, Gilead, Pfizer, Bristol Myers Squibb, Kite, Actinium, Arog, Immunogen, Arcellx, and Shattuck: Consultancy, Other: Advisory Role; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Gilead, Immunogen, Pfizer, Bristol Myers Squibb, Trovagene, Servier, Novimmune, Incyte, Hanmi, Fate, Amgen, Kite, Novartis, Astex, KAHR, Shattuck, Sobi, Glycomimetics, Trillium: Research Funding. Pemmaraju:stemline: Consultancy; abbvie: Consultancy; immunogen: Consultancy; mustangbio: Research Funding; incyte: Consultancy; novartis: Research Funding; pacylex: Consultancy, Research Funding; samus: Research Funding; daiichi sankyo: Research Funding; cellectis: Research Funding; cellularity: Research Funding. Yilmaz:Daiichi-Sankyo: Research Funding; Pfizer: Research Funding. DiNardo:Gilead: Honoraria; Bluebird Bio: Honoraria; Astellas: Honoraria; Servier: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Research Funding; GenMab: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Kura: Honoraria, Membership on an entity's Board of Directors or advisory committees; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria; Takeda: Honoraria; Astex: Research Funding; Cleave: Research Funding; Forma: Research Funding; Jazz: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Foghorn: Honoraria, Research Funding; ImmuneOnc: Honoraria, Research Funding; LOXO: Research Funding. Ravandi:Amgen: Honoraria, Research Funding; Syos: Consultancy, Honoraria, Research Funding; Astex/Taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Biomea Fusion, Inc.: Research Funding; Astellas: Consultancy, Honoraria, Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Novartis: Consultancy; Prelude: Research Funding; Xencor: Research Funding; AstraZeneca: Consultancy. Garcia-Manero:BMS: Consultancy, Honoraria, Research Funding; Aprea: Honoraria; Genentech: Honoraria, Research Funding; Gilead Sciences: Research Funding; Astex: Consultancy, Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Curis: Honoraria, Research Funding; Acceleron Pharma: Consultancy; Novartis: Honoraria, Research Funding. Kadia:AstraZeneca: Research Funding; Astellas: Research Funding; cellenkos: Research Funding; Delta-Fly: Research Funding; cyclacel: Research Funding; Regeneron: Research Funding; Glycomimetics: Research Funding; Astex: Honoraria; Iterion: Research Funding; Ascentage: Research Funding; Pfizer: Research Funding; Servier: Consultancy; JAZZ: Consultancy, Research Funding; Novartis: Consultancy; BMS: Consultancy, Research Funding; Agios: Consultancy; Genentech: Consultancy, Research Funding; Genfleet: Research Funding; PinotBio: Consultancy; Amgen: Research Funding; Abbvie: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal